CHILDREN'S NATIONAL RESEARCH INSTITUTE ACADEMIC ANNUAL REPORT 2021
Spotlight
Agreement brings mobile rare disease diagnostic platform to global market, with an emphasis on positive social impact
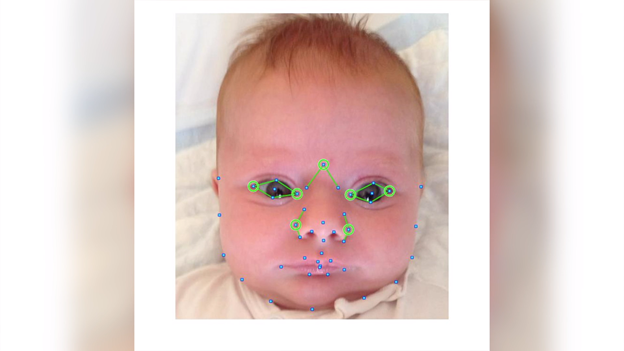
Children’s National Hospital has entered into a licensing agreement with Breakthrough BioAssets for mGeneRX, a patented pediatric medical device technology using objective digital facial analysis software for the early and non-invasive screening of dysmorphic genetic diseases. The technology was developed by Children’s National to provide a more advanced diagnostic tool for regions of the world with limited access to geneticists or genetic testing. MGeneRx is a spinoff from BreakThrough BioAssets LLC, a life sciences technology operating company focused on accelerating and commercializing new innovations, such as this technology, with an emphasis on positive social impact.
The objective digital biometric analysis technology was developed by a multidisciplinary Children’s National team led by Marius George Linguraru, D.Phil, M.A., M.Sc., of the Sheikh Zayed Institute for Pediatric Surgical Innovation and Marshall Summar, M.D., director of the Children’s National Rare Disease Institute (CNRDI). Research studies conducted in conjunction with the National Human Genome Research Institute at the National Institutes of Health further enhanced the technology’s development. In recent years, research has shown the potential of this technology to detect, with a 90 percent accuracy, early diagnosis of 128 genetic diseases across pediatric subjects in 28 countries. These diseases include DiGeorge syndrome (22q11.2 deletion syndrome), Down syndrome, Noonan syndrome and Williams-Beuren syndrome.
The application utilizes artificial intelligence (AI) and machine learning to analyze biometric data and identify facial markers that are indicative of specific genetic disorders. Physicians capture biometric data points of a child's face in real time within the platform, where it scans facial biometric features to determine the potential presence of a genetic disease, which can often be life-threatening without early intervention.
“We are delighted to enter into this licensing agreement through Innovation Ventures, the commercialization arm of Children’s National Hospital, which seeks to move inventions and discoveries from Children’s National to the marketplace to benefit the health and well-being of children. Our mission is to add the ‘D’ in development to the ‘R’ in research to accelerate the commercialization of our intellectual property,” said Kolaleh Eskandanian, Ph.D., M.B.A., P.M.P., vice president and chief innovation officer at Children’s National. “It is through partnerships with startups and the industry that we can achieve this goal and thus we highly value this new partnership with MGeneRx Inc. The acceleration and commercialization of this objective digital facial analysis technology will not only help diagnose rare genetic disorders – it will also allow for earlier interventions that improve the quality of life for the children living with these conditions.”
MGeneRx, Inc. will focus on cultivating additional capital to support the commercialization of the objective digital biometric analysis technology, with the goal of helping to bridge the gap in diagnosis and early intervention of rare genetic disorders in developing countries around the world. Additional data collected through the expanded use of the technology will help to further develop the application and expand its capabilities to identify and diagnose additional rare genetic conditions.
"The social impact of this technology cannot be underestimated within higher, middle and especially lower income nations around the world, where there is a high prevalence of rare genetic conditions but a severe lack in the specialty care required to diagnose and treat them,” says Nasser Hassan, acting chief executive officer of MGeneRx Inc. “We are excited about this licensing agreement with Children’s National Hospital and the opportunity to enhance this technology and expand its application to populations where precision medicine and the earliest possible interventions are sorely needed in order to save and improve children’s lives.”
The licensing agreement was arranged by Children’s National Innovation Ventures, which is focused on the commercialization of impactful new pediatric medical device technologies and therapies to advance children’s health care. Created to further catalyze the ongoing translational research of the Children's National Research Institute (CNRI) as well as inventions by hospital clinicians, the hospital’s Innovation Ventures focuses on four core pillars to advance pediatric medical device technologies including a Biodesign program, partnerships and alliances to augment internal capacity, seed funding to de-risk technologies and validate market and clinical relevance, and back-office operation to manage intellectual property and licensing activities. Since 2017, Children’s National intellectual property has served as the basis for over 15 licensing or options agreements with commercial partners.
Dr. Eskandanian added that access to an array of experts and resources for pediatric innovators is one of the aims of the Children’s National Research & Innovation Campus, the first-of-its-kind focused on pediatric health care innovation, with the first phase currently open on the former Walter Reed Army Medical Center campus in Washington, D.C. With its proximity to federal research institutions and agencies, universities, academic research centers, as well as on-site incubator Johnson and Johnson Innovation – JLABS @ Washington, DC, the campus provides a rich ecosystem of public and private partners, which will help bolster pediatric innovation and commercialization.