CHILDREN'S NATIONAL RESEARCH INSTITUTE ACADEMIC ANNUAL REPORT 2022
Spotlight
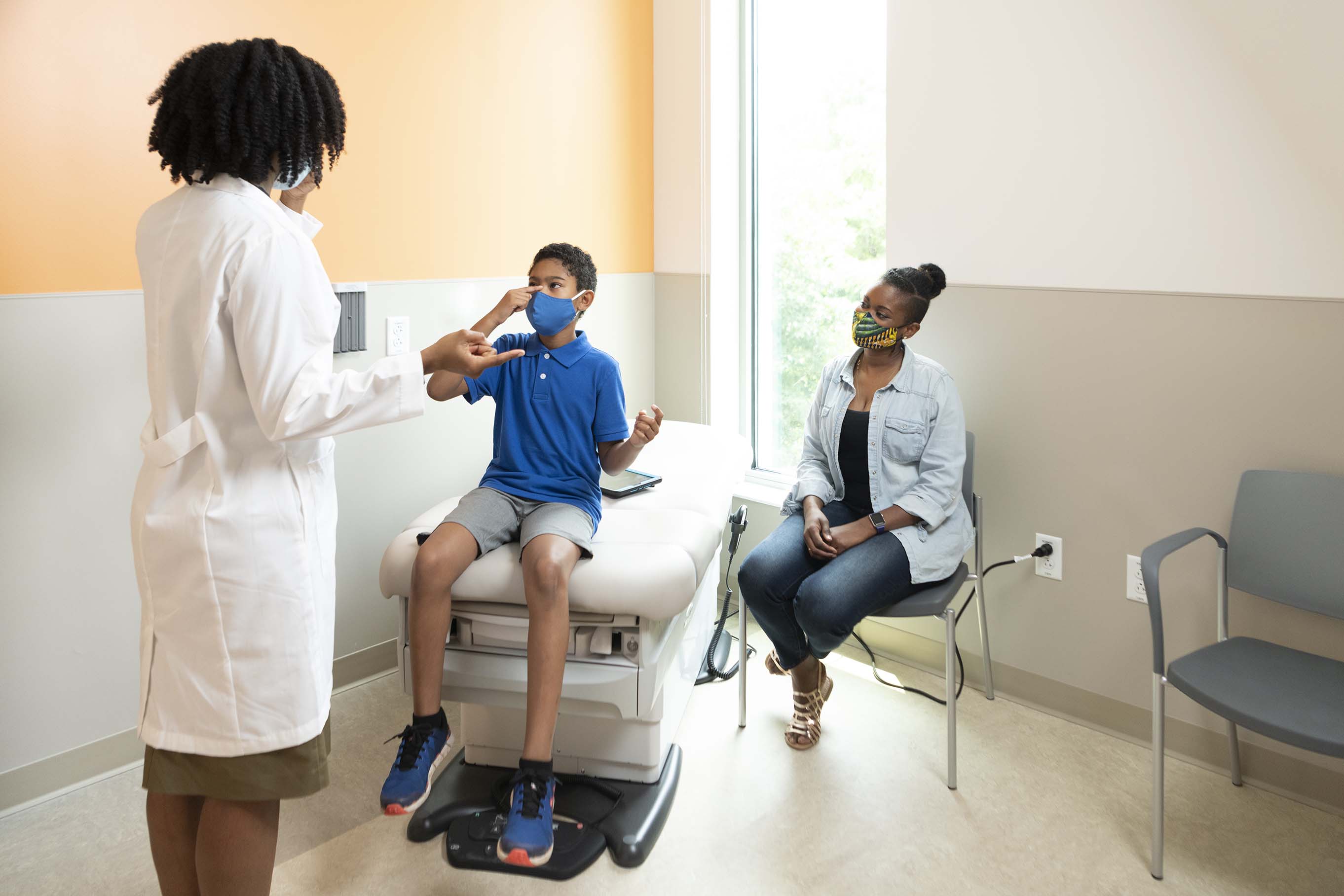
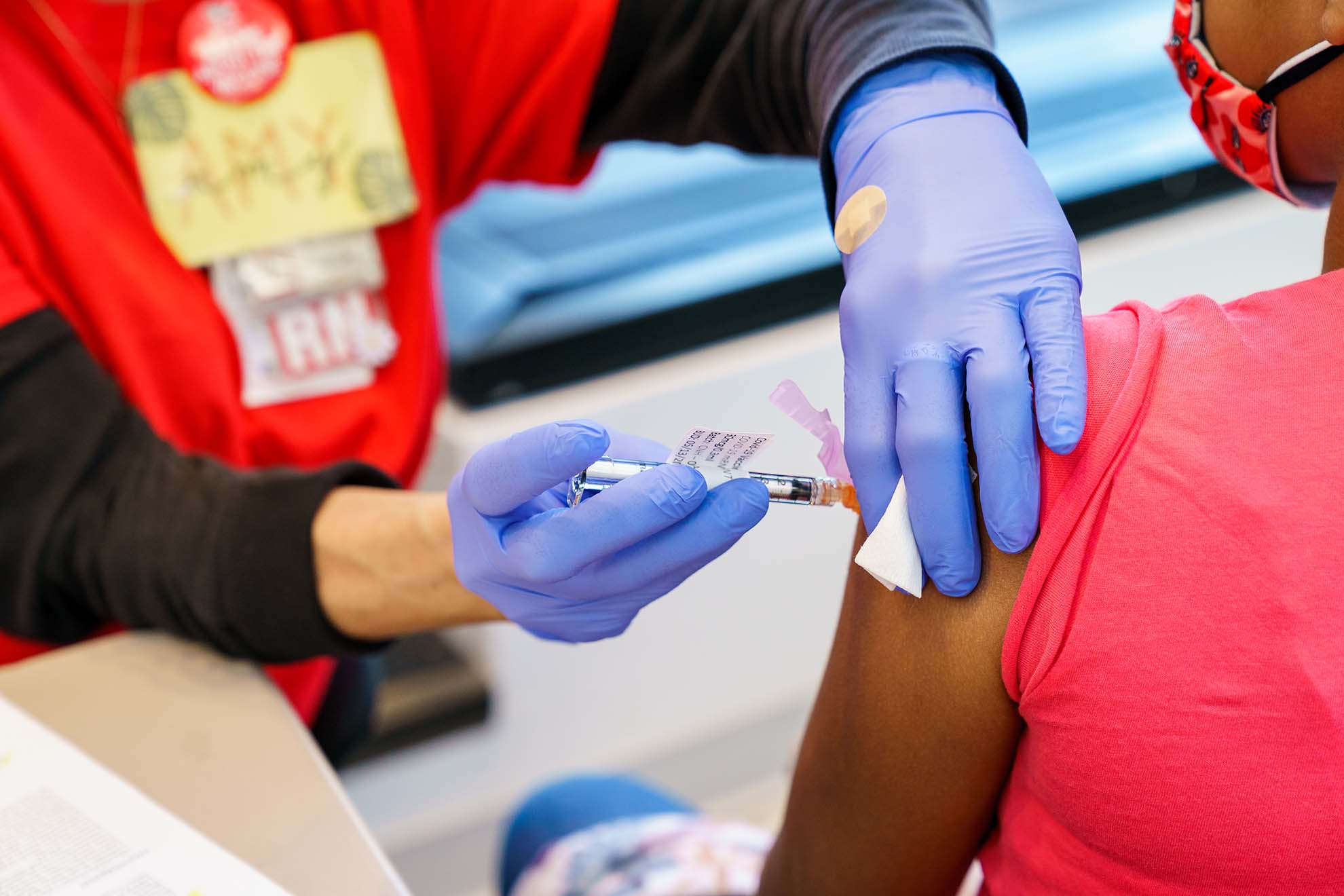
Quickly Overcoming Obstacles to Become a Top Recruiter for Young Children in the Pfizer COVID-19 Vaccine Trial
Vaccine trials generally require special resources to enroll and manage participants, and most institutions usually run more than one trial at a time. Children’s National Hospital did not have an established vaccine trials unit, which put us at a great disadvantage in competing with other mature centers to be accepted to participate in a COVID-19 vaccine trial. In 2021, we were fortunate to finally have our proposals accepted by both Pfizer and Moderna and ended up choosing to go with Pfizer. We were the last center allowed into the study worldwide last year.
Many of the centers in the study had already been participating in adult trials of the Pfizer COVID-19 vaccine.
The late start, the challenges everyone faced with conducting research during a pandemic and the unique circumstances of this particular trial in which centers were required to report information daily, after each participant event in the trial, made this even more difficult for us. Thankfully we had institutional leadership behind us, and even though we lacked vaccine trial experience, the staff in the clinical research unit were all very highly skilled in clinical trials in general and especially working with children and parents. Our first participants received injections on June 7, 2021.
By mid-September, we were doing well, and that is when we were told that we ranked sixth worldwide in enrolling children in the 6–23-month-old age group, usually the hardest group to recruit. All other centers in the top 10 enrollment were mature vaccine trial units, so this was quite the accomplishment.
However, as the need for more participants grew, our capacity was at its limit. This further highlighted the potential and opportunity to continue building for growth here at the Children’s National Research Institute.
We did have the resources to continue the trial for our enrolled subjects to include the booster dose phase that was added in January 2022. By the end of June 2022, all our trial participants received their boosters. The trial itself will continue for at least 12-18 months more, with telephone follow-up and assessment of new illnesses via telehealth visits.
Bernhard L. Wiedermann, M.D., M.A., attending in Infectious Diseases, says that “we are all very proud of what we’ve accomplished, with special thanks to hospital leadership, Lisa M. Guay-Woodford, M.D., the staff of the clinical research center and most importantly our participants and their families.”